A Fluoride Ion Is Isoelectronic With Which of the Following
Which element has the same electronic configuration ie isisoelectronic as these elements. They may have different numbers of protons and neutrons but the number of electrons will be the same.
What Is Isoelectronic With Fluorine Quora
Now we can see that the neon atom the magnesium ion the aluminum ion and the fluoride ion all have the same number of electrons.
. F- would have 8 valence electrons. The atomic number of fluorine rmF is nine that means the number of electrons in fluorine is nine. Write the electronic configuration using an orbital energy.
1s2 2s2 2p6 is the electron configuration of the fluoride ion. I- is isoelectronic with the noble gas Xenon. Selenium has 34 electrons and a gain of 2 electrons will form 36 electrons.
N2- would have 7 valence electrons. Is Caesium a metal. Which of the following ions is isoelectronic with the noble gas argon.
33 Which of the following anions is isoelectronic with argon. Accordingly the atomic number of O is 8 and of F is nine. The correct option is B.
Mg2 always has one electron around it. Ca2 is isoelectronic with the noble gas Argon. Oxygen atom has 8 electrons and O 2- ion will thus have 8 2 10 electrons.
Upon reduction the fluorine atom forms fluoride which has 8 valence electrons and is isoelectronic with a Noble Gas which one. A 1s2 2s2 2p4 B 1s2 2s2 2p5 C 1s2 2s2 2p6 D 1s2 2s2 2p6 3s1 E none of the above. So rmO2 - is isoelectronic with neon.
For example - Fluoride ion F- and Sodium ion are isoelectronic species. Write the electronic configuration of the following ions. And thus the neutral atom has 7 valence electrons.
Oxide sodium and magnesium ions also all have 10 electrons in this configuration so they are all isoelectronic with each other forming an isoelectronic series. The electron configuration of a fluoride ion F is. The species isoelectric to are Kr.
This electron is added to the 2p subshell. B Strontium fluoride SrF 2 Sr 2 and 2F. O- would have 7 valence electrons.
F- and Na are isoelectronic species. Thus it has an electronic configurations of 1s 2 2s 2 2p 6 3s 2 3p 6 and is isoelectronic with H 2 S. Isoelectric means that two atoms have the same number of valence electrons.
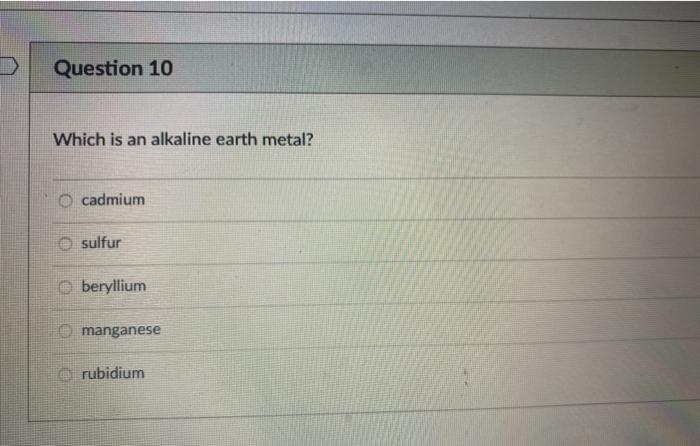
Answer to Solved 33 Which of the following anions is isoelectronic. The Bromide ion has 36 electrons. Chloride ion magnesium ion lithium ion oxygen sodium Question 10 Which is an alkaline earth metal.
Caesium is a soft gold-coloured metal that is quickly attacked by air and reacts explosively in water. Now the interesting part. Fluoride ion sodium ion and nitride ion.
In lithium chloride Li has no electrons around it. In ionic compounds containing fluoride. After gaining an ion in the valence electron fluoride ion will have a total of 10 electrons which corresponds to the total number of.
A series of atoms ions and molecules in which each species contains the same number of electrons but the different nuclear charge is called isoelectronic series. 122 lonic Bonds 34 Draw the electron dot formula for ammonia NH3. BYJUS Online learning Programs For K3 K10 K12 NEET.
Therefore these two atoms are not isoelectronic to F- as they both have one less valence electron. It has a complete octet and is isoelectronic with neon. Hence option A is correct.
Iso means equal and isoelectronic means the same number of electrons. The ions O2- F- Na Mg2 and Al3 are isoelectronic. Since carbon monoxide and nitrogen molecules have the same number of electrons therefore CO and N_2are isoelectronic.
This leads to the conclusion that O has to have 8 electrons to compensate for those protons and F. So Cs is isoelectronic with Xe and S 2 is isoelectronic with Ar. The Krypton Kr also has 36 electrons.
A F-B Na C Mg2 D Al3 E all of the above. Asked Dec 17 2018 in Chemistry by pinky 743k points classification of elements and periodicity in properties. Question 5 5 pts Fluoride ion is isoelectronic with.
Of course the elemental form is bimolecular. Therefore the F ion has 10 electrons and its electronic configuration is 1s2 2s2 2p6. Fluorine gas exists as diatomic molecule F 2 with a total of 2 9 18 electrons.
Which of the following statements regarding Lewis dot symbols of ions is false. Which of the following ions is isoelectronic with the noble gas neon. We review their content and use your feedback to keep the quality high.
A nitride ion B sulfide ion C bromide ion D all of the above E none of the above Section. So option B is incorrect. So rmFis not isoelectronic with neon.
View the full answer. Isoelectronic species are the ones that have the same number of electronsBy gaining the electrons or losing electrons the same electronic configuration can be achieved. What is the electron configuration for a fluoride ion F-.
F- Mg2 are isoelectronic with the noble gas Neon. In rmO2 - due to gaining of two electrons the number of electrons is ten. Write the electronic configuration of two ions that are isoelectronic with argon3.
O and F are two consecutive atoms in the periodic table which means that they differ structurally by one proton. For example fluoride ion is isoelectronic to neon in that F and Ne each have 10 electrons in the configuration 1 s 2 2 s 2 2 p 6. In magnesium sulfide S2 has eight electrons.
5-Fluoride ion consists of ten electrons as normal fluorine contains nine electrons and in its ionic form it t. Those ions atoms or molecules which have the same number of electrons are known as isoelectronic species. When the number of electrons is the same in different species that can be atoms molecules or ions then they are known as isoelectronic species.

Answered Write The Chemical Formula For Each Compound Described Writing Chemical Formula Chemistry
What Is Isoelectronic With Fluorine Quora

Solved Question 5 5 Pts Fluoride Ion Is Isoelectronic With Chegg Com

Solved Question 3 1 5 Points Match The Following Fluoride Chegg Com
What Is Isoelectronic With Fluorine Quora
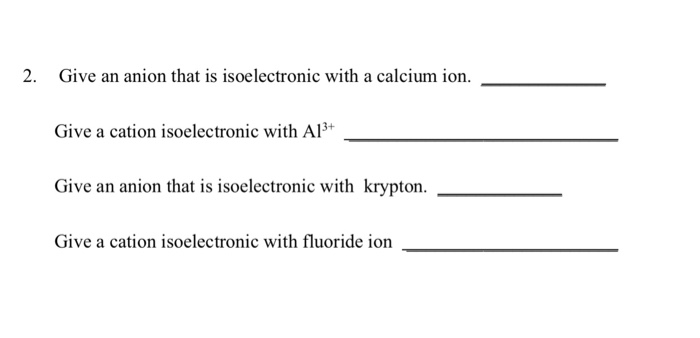
Solved 2 Give An Anion That Is Isoelectronic With A Calcium Chegg Com

Which One Is Isoelectronic So32 Co32 No3 Or Cn N2 C22 Isoelectronic Species Youtube
What Is Isoelectronic With Fluorine Quora
Which Has Larger Size Among Sodium Cation Fluoride Ion And Neon Atom Quora

Size Of Isoelectronic Species Ii Which Is Smaller In Size O2 F Mg2 Youtube
What Is Isoelectronic With Fluorine Quora

Question Video Identifying The Species That Is Not Isoelectronic With The Others In A List Of Atoms And Ions Nagwa

Solved 33 Which Of The Following Anions Is Isoelectronic Chegg Com
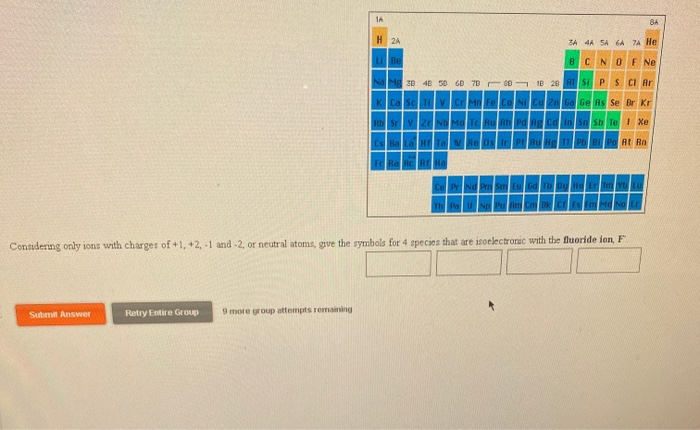
Solved 1a 84 H 2a Sa 44 54 6a 7 He Be B C N O F Ne Na Z 4 5 Chegg Com
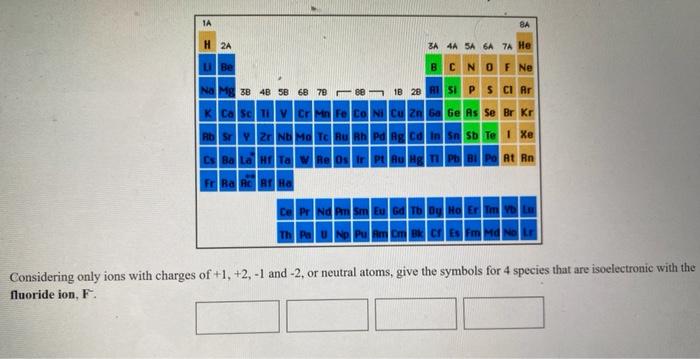
Solved 1a 8a H2a Za 4a 5a 6a 7a He Be Bc N O F Ne Na 38 48 Chegg Com
What Is Isoelectronic With Fluorine Quora

Solved Which Of The Following Anions Is Isoelectronic With Chegg Com

Solved D F Fluoride Ion 13 Which Of The Following Are Chegg Com
Comments
Post a Comment